The Food and Drug Administration on Friday approved a new gene-editing therapy to treat sickle cell disease, a debilitating blood disorder that affects at least 100,000 Americans, most of whom are Black. The treatment, called Casgevy, is the first approved medicine in the U.S. to use the groundbreaking gene-editing tool CRISPR to alter DNA.
The new therapy, from Vertex Pharmaceuticals and CRISPR Therapeutics, is, experts say, a stunning advancement in medicine. The treatment, however, also sparks mixed feelings among both Black sickle cell patients and doctors, who are concerned about potential side effects, costs and access to the therapy, will cost $2.2 million per patient, according to Vertex Pharmaceuticals.
Excitement about the treatment — which would eliminate the need for bone marrow transplants to cure the disease — is also colliding with some lingering feelings of distrust of the medical system among parts of the Black community. It’s the first time a therapy that uses CRISPR, which was invented in 2009, has been approved, and whether it causes long-term side effects is unknown.
“We are aware that there are a number of individuals who are still a little bit hesitant. But overall, we’re excited mainly because it’s another option,” said Derek Robertson, the president of the Maryland Sickle Cell Disease Association, whose two sons have sickle cell disease.
“This is a potential life-transforming therapy,” Robertson said.
‘We live in pain every day’
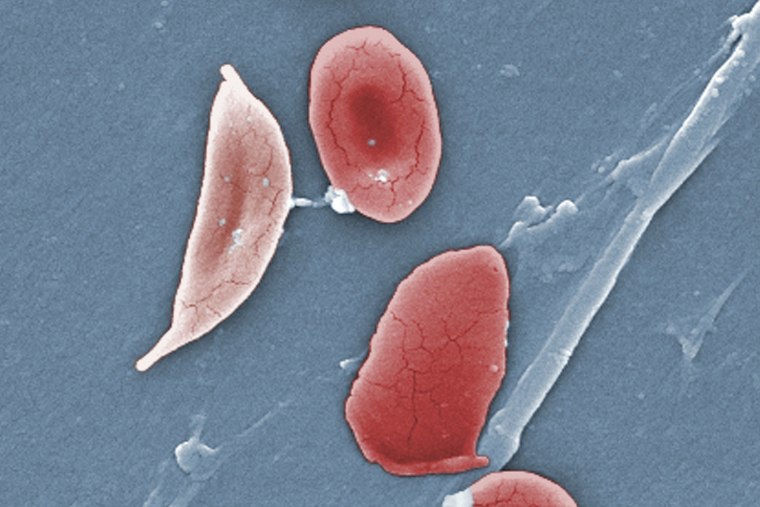
In people with sickle cell disease, red blood cells, which are normally disk-shaped, take on a crescent or sickle shape. That shape can cause blood cells to clump together, blocking blood flow and depriving tissues of oxygen, said Dr. Cecelia Calhoun, a hematologist-oncologist and the medical director of the Adult Sickle Cell Program at Smilow Cancer Hospital at the Yale New Haven Health System in Connecticut.
While sickle cell disease is most known for causing severe pain, patients can experience stroke, heart disease, organ damage and a dangerous condition called acute chest syndrome caused by blockages in the lungs, Calhoun said. The main symptoms of sickle cell disease are disruptive to people’s “quality of life and ability to just function as normal humans,” she said.
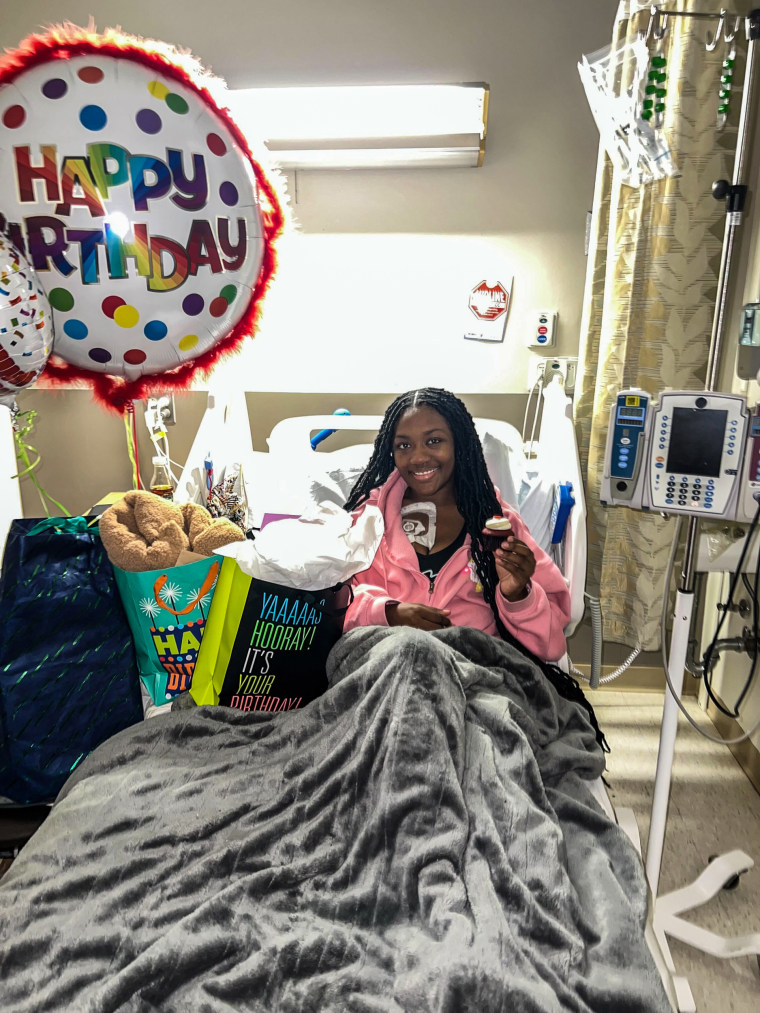
That has been the case for Kayla Smith Owens, 25, of Chesapeake Beach, Maryland, who started experiencing mild symptoms of sickle cell disease pain and fatigue until around age 12, during puberty. It wasn’t until she started college at Bowie State University in 2016 when “things got really, really bad,” she said.
“I had no college experience at all, because my time in college consisted of being in the hospital for seven days, coming home, catching up and then going right back to the hospital,” said Smith Owens, who graduated last year with a degree in criminal justice. “I couldn’t join clubs. I didn’t have time to make friends, because my time was spent in the hospital.”
Since she graduated, Smith Owens said, she hasn’t been able to work because she’s “always sick” and in extreme pain.
Even with medications and blood transfusions, sometimes her symptoms are “too far gone,” which requires immediate hospital care, she said.
“We live in pain every day,” Smith Owens said about the reality of many sickle cell patients. “There’s not really a day where I wake up and feel nothing.”
Like Smith Owens’, Obi Ariguzo’s sickle cell disease symptoms have worsened with age. When he was a teenager, Ariguzo said, he got sick only once every four years. Now, at 39, he experiences a pain crisis at least once a month. Because of the disease’s effect on his bones, he had to undergo four hip replacements — which made it difficult for him to continue his work related to visual effects in the film industry.
“I couldn’t go back and walk all over set until I could actually move really freely without aid of a cane or crutches,” said Ariguzo, who lives in Austin, Texas. Ariguzo now works at the Texas Department of State Health Services, because the position “allows me to be at a desk and recuperate,” he added.

While Ariguzo tries not to let his disease affect him, he said it’s difficult to do so with the frequent onslaught of symptoms and hospitalizations. Ariguzo said he avoids work that is too strenuous, limits flying and doesn’t drink alcohol — which can all trigger a pain crisis.
“It’s all the complications that it causes that really is frustrating,” he said, “and you don’t know what the next one’s going to be.”
An arduous treatment
Casgevy works by editing the DNA in a person’s bone marrow stem cells, which make the body’s red blood cells, so that they no longer take on a sickle shape.
The treatment process is arduous, however, and it involves months of blood transfusions, followed by bone marrow extraction and chemotherapy. The bone marrow is sent to a lab where it is genetically edited. Before patients can get the drug, they must undergo chemotherapy to wipe out any remaining stem cells that could make sickle-shaped cells.
Dr. Jeffrey Glassberg, the director of the Mount Sinai Center for Sickle Cell Disease at the Icahn School of Medicine in New York City, said gene therapy drugs can be incredibly impactful in treating sickle cell disease. Some of his patients, he said, participated in the Casgevy clinical trial, as well as trials for another gene therapy, from Bluebird Bio, that was also granted FDA approval on Friday.
“With the patients I have who’ve had this therapy, they have normal blood counts,” Glassberg said. “They run 3 miles a day. They don’t have sickle cell disease anymore.”
Still, not everyone may be on board. Although about 1,500 people are treated for sickle cell in Mount Sinai’s program, Glassberg estimated that less than 10% will opt to get the new treatment, in part because of some of the risks that come with the chemotherapy that patients must get as part of the treatment. The risk, he added, “really has nothing to do with gene therapy.”
“You have to get quite a bit of toxic chemotherapy, which raises the risk that you could develop secondary cancers later on,” said Glassberg, who isn’t involved with the clinical trials. The chemotherapy also raises the risk of infertility, he said.
Cost concerns
Another major concern for doctors and patients is access. Casgevy will cost $2.2 million per patient, according to Vertex Pharmaceuticals. A 2021 analysis by NORC, an independent research organization affiliated with the University of Chicago, identified 52,524 people with sickle cell disease who were enrolled in Medicaid in 2021 and found that Black Medicaid enrollees made up 87% of Medicaid enrollees with sickle cell disease.
Conversations around sickle cell gene therapies should lead with the notion of how effective the therapies will be for patients rather than the cost, because it changes the “focus and the perspective,” said Robertson, of the Maryland Sickle Cell Disease Association, whose wife works in patient advocacy at Vertex.
“The perspective is once this therapy works, we need to get it to the people who need it, and then the cost is something that folks will work on,” he said.
Glassberg said there are several ways coverage of the treatment could play out. It’s possible that insurance companies could limit coverage to patients who meet the exact criteria of those in the clinical trial — for example, the trial didn’t enroll any patients older than 35. That could “filter out and justify covering the drug for few people,” he said.
On the flip side, Glassberg said he has also seen insurance companies in states like New York cover expensive drugs to treat sickle cell disease, including the drug voxelotor, which he said costs $125,000 per year.
“My experience has been nobody really knows what’s going to happen until it just hits the market and then stuff starts to happen,” he said. “Then we sort of figure out how to make it work.”
Still, doctors are overall optimistic about the treatment’s ability to drastically change the lives of sickle cell disease patients.
The therapy prevents acute pain crises and “hopefully averts chronic complications to develop over time,” said Dr. Jean-Antoine Ribeil, a hematologist and the clinical director at the Sickle Cell Disease Center at the Boston Medical Center. If patients respond well to the treatment, it “has the potential to be life-changing.”
“I’ve had some very emotional conversations with patients who are hopeful for a treatment that works,” Ribeil said in an email.
Ariguzo said the approval is “fantastic news.” He has tried several medications and treatments over the years, including blood transfusions and medicines like hydroxyurea and Adakveo — a drug taken once a month to help limit the number of pain crises. All have proven unsuccessful.
“At this point, if anything can help kind of limit the amount of pain crises I’m having with how frequently they’re happening, I’m all up for it,” Ariguzo said. “I feel like I’ve tried everything at this point.”
Keeping patients out of the dark
Doctors are working hard to ensure that their patients are well-informed about the gene-editing treatment to ease any possible concerns or hesitations.
Calhoun, of Smilow Cancer Hospital, said she walks her patients through the gene therapy process, along with potential risks and benefits. She also opens a safe space for conversation and treats her patients as she would a relative, which helps establish trust, she said.
“I tell my patients, ‘I get to see you an hour today in a clinic visit, but the other 23 hours of the day and six days a week, you’re with yourself,’” Calhoun said. “‘So I have to use this time to give you the information you need to make an informed decision,’ and that’s exactly what I do.”
She also talks to patients about the importance of participating in clinical trials and how doctors make scientific discoveries.
A 2015 study from the Society for Clinical Trials found that 25 of the 174 clinical trials for sickle cell were terminated or withdrawn because of low enrollment. Additional data from a 2016 study in The American Journal of Preventive Medicine found that mistrust is one factor contributing to low participation numbers, especially among Black males, who are underrepresented in biomedical research.
The study cites the Tuskegee Syphilis Study, which involved 600 Black men — two-thirds of whom had syphilis — who were mostly poor and illiterate sharecroppers. From 1932 to 1947, several of the participants in the study died from the disease, and their wives and children became infected. When it was clear in 1943 that penicillin could treat syphilis, the participants weren’t offered treatment.
The study was later labeled “ethically unjustified,” because protocol that was usually applied to human subjects involved in scientific research “was either ignored or deeply flawed,” according to Tuskegee University. The men were also not informed of the study’s name and purpose and the possible risks of participating.
Glassberg said patients of color have “plenty of reason” to mistrust the medical system not only because of the historical keynote horrors that affected them, seen in cases like that of Henrietta Lacks — whose cells were harvested without her consent — but also because of the mistreatment and pervasive stigmatization that Black sickle cell patients still experience today.
While a majority of patients in his program don’t display feelings of mistrust, Glassberg said he and his colleagues have done a lot of work to “lay the foundations of trust and gain confidence of our patients.” That includes offering social workers and community support groups and having a group of patients that “approves our research studies,” he said.
While Ariguzo said he’s “still trying to weigh what the risks are with the gene therapy,” it’s not enough to stop him from wanting to have the treatment. Being free of sickle cell disease would allow him to plan long-term, because, “in my mind, I’m trying to just make it to the next month or two without major interruptions,” he said. He also said getting the treatment would improve his mental health.
“It would relieve so much anxiety and also depression stuff, too,” he added. “I feel like it would make me a lot happier.”
Smith Owens said she’s well aware of the risks the new gene therapy may pose and has been well-informed about the treatment process.
In 2020, she went to the National Institutes of Health to undergo a bone marrow transplant, but the plan with her donor fell through. She said she has been waiting for the FDA to approve the gene therapy for “a very long time.” And for her, the decision couldn’t come any sooner.
“I feel like it would be huge and change my life tremendously,” she said. “The only thing that I want to do in life is continue my education and be able to get a job. Like, I know that seems so small, but that’s literally all I want to do.”






