Health regulators around the world are sounding the alarm about counterfeit versions of Ozempic. Fake versions of the hugely popular diabetes drug that is often prescribed off-label for weight loss have been reported in over a dozen countries, including the United States, and have also caught the attention of the World Health Organization.
“The thing about this product is that it’s very popular, people are going to use it,” said Timothy Mackey, a professor of global health at the University of California, San Diego who studies counterfeit drugs. “Global regulators are trying to shut down the counterfeit drugs wherever they may go. Americans or anyone around the world are susceptible to counterfeits.”
Last month, the WHO’s Member State Mechanism on Substandard and Falsified Medical Products discussed counterfeit semaglutide products, said WHO spokesperson Daniel Epstein. Semaglutide is the active ingredient in Ozempic.
The group encouraged member countries to “take appropriate action in the face of the recognized threat,” Epstein said.
In the U.S., the Food and Drug Administration said last week that it seized thousands of counterfeit units of the drug.
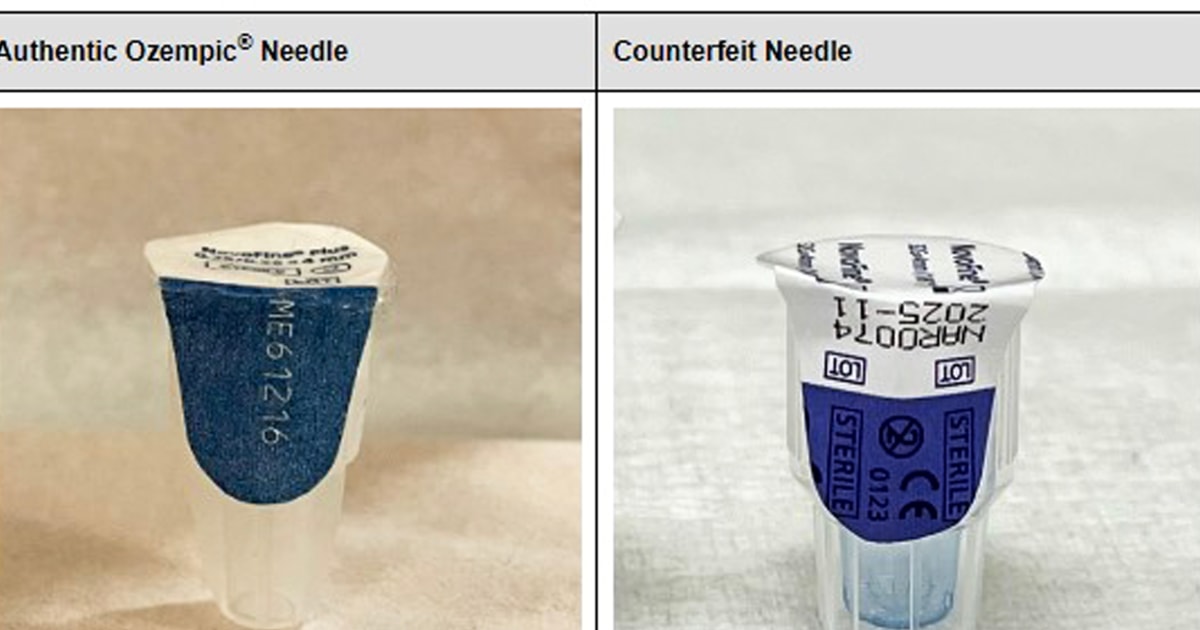
The FDA said it was working with drugmaker Novo Nordisk, which holds the patent on Ozempic, to test the counterfeit drugs to find out what’s in them. They’ve already determined that the needles that came with the seized injectable medication are counterfeit and might not be sterile, the agency said. The labeling, packaging and prescription information given to patients and doctors were also falsified.
To date, no one has been seriously hurt by the counterfeit medications, the FDA said. The agency is aware of five adverse events related to the fake products, though all of them were consistent with known side effects seen with real Ozempic, including nausea, vomiting and diarrhea.
In October, the European Medicines Agency said fake versions of Ozempic had been found at wholesalers in the European Union and the United Kingdom. The counterfeit pens, labeled in German, were detected when the serial numbers on the packages were scanned and came up as inactive, the EMA said.
Several people in Austria were hospitalized after using the counterfeit products, Austrian regulatory authorities said in a separate statement. Serious side effects included hypoglycemia and seizures, an indication, authorities said, that the counterfeit products contained insulin instead of semaglutide. German authorities confirmed in November that a batch of counterfeit Ozempic contained insulin.
It’s unclear whether the counterfeit Ozempic found in the U.S. supply chain is linked to the fake drugs discovered in other countries, Mackey said.
The FDA declined to comment beyond its statement last week, stating that the investigation is ongoing. Jamie Bennett, a spokesperson for Novo Nordisk, also declined to comment.
Last week, the company said that the seizure of counterfeit drugs took place in warehouses outside its authorized supply chain.
The Partnership for Safe Medicines, a nonprofit coalition of supply chain producers and distributors, has been tracking cases of counterfeit Ozempic and other weight loss drugs across the world.
At least 16 countries have encountered fake Ozempic, said the group’s executive director, Shabbir Imber Safdar.
“The fake products aren’t exactly identical, but close enough that they don’t look obviously fake,” he said.






