The only approved treatments for Alzheimer’s disease are medications with limited effectiveness and a risk of severe, sometimes deadly, side effects. That’s why scientists search for therapies that could stop the disease, especially ones that don’t involve drugs.
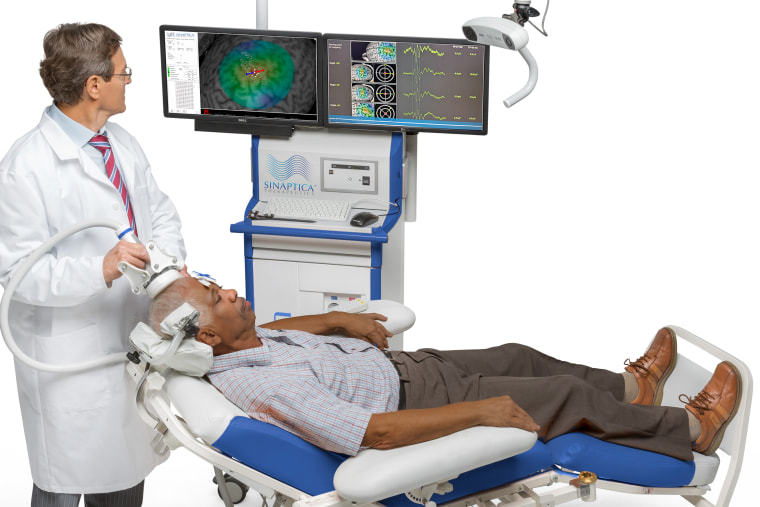
One experimental therapy may slow the progression of symptoms, a small preliminary study suggests. Using a transcranial magnetic stimulation (TMS) device, which is widely used to treat depression and other mental illnesses safely, researchers were able to target a key brain network that is involved in storing memories and is typically hit hard by the disease, according to the report presented Thursday at the Clinical Trials in Alzheimer’s Disease meeting in Madrid.
Researchers found that when the device was aimed at the right spot in the brain, it could slow the development of symptoms, such as memory loss, compared to an inactive treatment.
In Alzheimer’s, nerve cells in the brain at some point start to dysfunction, leading to the debilitating symptoms of memory loss. Previous research has indicated that the accumulation of two aberrant proteins, beta-amyloid and tau, damage the ability of neurons to form new connections and to maintain existing ones, said Dr. Giacomo Koch, a professor of human physiology at the University of Ferrara and one of the co-founders of Sinaptica, the Cambridge, Massachusetts-based company currently developing the therapy.
“The goal is to restore connections between neurons by enhancing activity in certain areas relevant to the disease,” Koch said in a Zoom interview with NBC News. “This therapy is like training for the neurons.”
The idea is that just as exercise strengthens muscles, the electrical signals generated by the TMS might enhance the ability of neurons to make connections with one another.
About 6.9 million people have Alzheimer’s in the U.S. That number could reach 13.8 million by 2060, according to the Alzheimer’s Association.
The new study, a phase 2 clinical trial, included 32 volunteers with Alzheimer’s disease, aged 56 to 88 at the start of the study, who were followed for 52 weeks. Sixteen of the participants who got the treatment were women.
At the start, the researchers determined the exact spot in the brain’s default mode network, which is involved in storing memories of life events, that would benefit the most from electrical stimulation by using TMS to “ping” various sites. When the right spot was nudged into action by the electricity, a signal would spread through the network like the ripples seen when a stone is tossed into a body of water.
Next, 18 of the volunteers received weekly 20-minute sessions with the TMS while 14 received so-called sham treatments, in which participants were treated as if they were getting TMS therapy, but without the device being turned on, to rule out the placebo effect. The TMS device was crucial to the research because it allowed electrical signals to be generated in the brain without any sensation.
“It would be almost impossible to use an electrical current because it would be very painful,” Koch said. “In this case we can use very powerful magnetic fields, which are very well tolerated and safe to induce strong electrical currents in the brain.”
Side effects were fairly uncommon and included mild headaches, skin discomfort and neck pain.
When the two groups were compared using standard cognitive tests, the researchers found the patients receiving TMS therapy had a 44% slower rate of symptoms worsening.
To put that in perspective, two of the newer medications, lecanemab and donanemab, have been shown to moderately slow a decline in memory and thinking abilities — by 27.1% and 22.3%, respectively. The treatments are monoclonal antibody infusions, given every two or four weeks and are costly — $26,500 to $32,000 per patient per year. Both are associated with an increased risk of brain swelling and microhemorrhages.
What’s more, during the yearlong TMS trial, participants receiving the experimental treatment showed little decline in their abilities to perform the activities of daily living. “That’s important not only for the patient, but also for caregivers,” Koch said.
Koch and his colleagues are currently planning a phase 3 trial, which would be needed for Food and Drug Administration approval.
Dr. Irina Skylar-Scott, a cognitive neurologist and a clinical assistant professor at Stanford University’s Center for Memory Disorders, said the procedure in the study shows promise. “As a field we are all excited about novel mechanisms and novel pathophysiological targets.”
However, there are significant limitations to the research. The trial size is small and involved only one location.
“The next step is to do a phase 3 trial across multiple centers to see if this bears fruit,” said Skylar-Scott, who was not involved in the research. “If it works it will be very exciting.”
The findings are “very, very preliminary,” said Dr. Lawrence Honig, a professor of neurology at the Columbia University Irving Medical Center. “On the face of it, if you look at the numbers, it did better on a number of scales compared to the sham treatment — that’s good. But as in any study, the devil is in the details.”
This is a small, single center study, Honig said. “A multicenter trial would offer a little more hope of generalizability,” he added, meaning it could apply to a broader group of people. Honig was not part of the new study.
Honig would also like to see measurements of biomarkers in a future study, such as blood tests and brain scans to determine whether there are actual improvements in the disease, shown, for example by reductions in tau and/or amyloid in the brain, along with reducing symptoms.
As for what he would tell his patients: “On the basis of these results you can’t say much about the utility of these treatments.”
The idea behind the new research “is very cool,” although limited by the small number of patients, said Dr. Ryan Darby, an assistant professor of neurology and director of the frontotemporal dementia clinic at the Vanderbilt University Medical Center.
Another issue: It’s not yet clear whether this method is something other centers will be able to easily adopt, said Darby, who was not part of the TMS research. “But I think the results are exciting and worth pursuing.”






